Alzheimer’s disease (AD) is the most common form of dementia causing gradual cognitive impairment and memory loss, often beginning with an individual’s short term memory. There are currently around 900K people living with the disease in the UK and an estimated 54 million people with any form of dementia globally. There is no known cure for the disease, but there are medical treatments available for a variety of symptoms, including memory loss, confusion and other behavioural effects. There is presently a large amount of ongoing research into the cause and treatment of AD, with a selection of new pharmaceutical products seeking regulatory approval and marketing authorisation.
As a result of the prevalence of the disease and the ever-advancing research, healthcare professionals (HCPs) across the globe use social media to raise awareness of symptoms, offer supporting advice and discuss up-and-coming treatments in the field. CREATION Pinpoint® identified almost 70K English language posts about Alzheimer’s over the past year posted by 14K HCPs worldwide. The most significant driver in the conversation was the controversial FDA approval of Biogen’s Aduhelm (aducanumab), the first novel therapy approved for the disease since 2003.
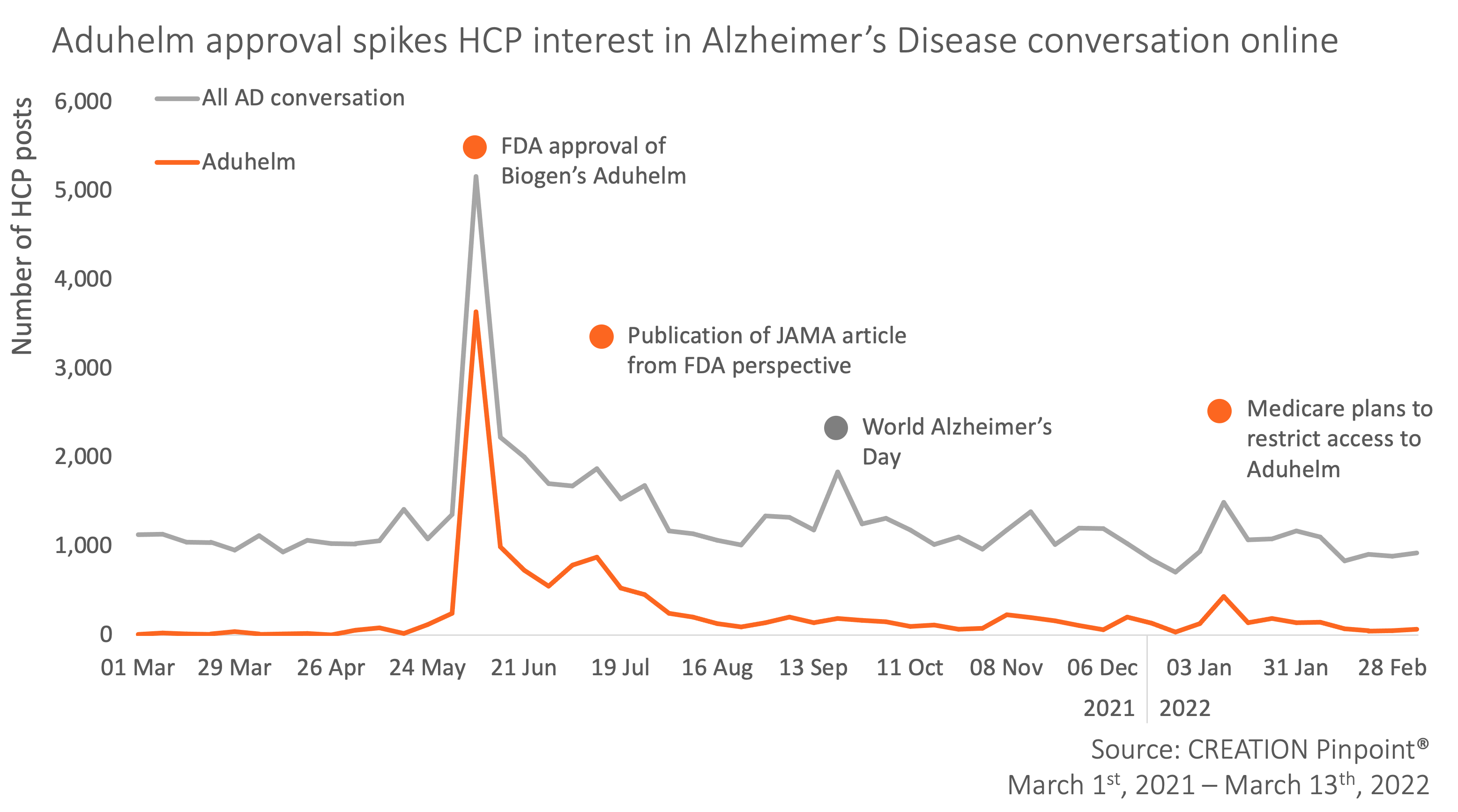
Throughout the year, a number of events spiked the interest and conversation of HCPs as the new product was scrutinised following its FDA approval.
Aduhlem’s journey to FDA approval
Prior to Aduhelm’s approval, it had had a number of failed and discontinued trials on account of safety concerns. Only a few months later, Biogen filed for regulatory approval with the backing of new analysis and a larger dataset. The presentation of this data at #CTAD19 was met with more questions than answers as HCPs remained cautiously optimistic of the impact this treatment would have for patients.
Great news for AD patients and the field in general! While I’m still skeptical of the clinical significance, I hope this is the first step towards a cure
In shocking reversal, Biogen to submit Alzheimer's drug for approval https://t.co/yjdUfVI5Bn
— Pieter Beerepoot (@beereppc) October 22, 2019
Before the approval there was already a divided sentiment, made more prominent by the views of significant organisations and advocacy groups. While the American Geriatrics Society urged the FDA not to approve the drug, the Alzheimer’s Association promoted aducanumab in a campaign called #moretime.
Must read: American Geriatrics Society urges FDA to NOT approve aducanumab for Alzheimers or MCI. The argument in their letter to FDA is compelling. Very much appreciate @AmericanGeriatrics taking a stand on behalf of the older adults we servehttps://t.co/LNV6v5JC92
— Ken Covinsky (@geri_doc) June 4, 2021
Wow. @alzassociation doing a full on biogen ad campaign for aducanumab called #moretime. Ad missing important info like the trials stopped early as it wasn’t working (& only showed small benefit in post hoc analysis of 1/2 trials). More herehttps://t.co/sYtbGDk4mE
— Eric Widera, MD (@EWidera) May 7, 2021
June 7th: Approval day
The week of the accelerated approval of Aduhelm saw a peak of over 5K HCP tweets dissecting and deliberating the news. HCPs were left confused by the decision since there hadn’t been “enough evidence to approve Aduhelm”.
HCPs called it a “dark day” as the approval came despite failed trials and disapproval from the FDA’s own scientific advisory panel. This contradiction by the advisory panel proved to be significant evidence to validate HCP reactions as a STAT News article recording the resignation of a panel member was shared over 100 times. The evidence appeared multi-faceted as HCPs cited the insufficient efficacy data, unreasonably high costs and worrying safety statistics.
New drug for Alzheimers approved by FDA after FDA's own scientific advisory panel advised against it. 10 out of 11 panel members concluded there was insufficient evidence this drug works.
This is crazy when evidence shows ketogenic diets benefit both prevention and treatment. pic.twitter.com/kFdZPQd4Vv— Paul Mason MD (@DrPaulMason) June 8, 2021
July 13th: FDA response
A month after the news of Aduhelm’s approval came to light, an article was published in JAMA Internal Medicine from the FDA’s Center for Drug Evaluation and Research (CDER) giving their perspective on the regulatory decision. The piece was introduced to “discuss the complexities of the data supporting the aducanumab application and the rationale for the FDA’s decision to grant it accelerated approval.”
A Family Medicine physician from New Haven, CT, Reshma Ramachandran, published a Twitter thread breaking down the key points of the article, warning her network not to read it unless they “want their blood to boil”.
So many issues with this piece in @JAMAInternalMed from @US_FDA officials (Dunn, Stein, & Cavazzoni) attempting to justify their poor choice in approving #aducanumab – don't bother reading it unless you want your blood to boil. I'll summarize instead: https://t.co/ddQ8EmDvoD 1/
— Reshma Ramachandran (@reshmagar) July 13, 2021
Dr Ramachandran cited discrepancies across phase I-III trials, suboptimal secondary endpoints and “most infuriating and saddening” a false hope for patients who have wondered when they’ll be able to receive the supposedly ineffective treatment.
One of the authors of the article, the director of the FDA’s Office of Neuroscience Billy Dunn, appeared central to the events of this approval and one HCP asked in a Twitter poll whether disciplinary action was warranted against him.
To those who followed the #BioGen #Aducanumab course of events with the @US_FDA , do you think a disciplinary action is warranted against Billy Dunn, director of neurology products for the FDA?#Alzheimer
— Ralph (@StuckInStock) June 9, 2021
Another HCP in Italy, Matteo Cesari, shared the FDA viewpoint to his network on Twitter, suggesting that “subjective feelings” had been prioritised over the data, which should not be the case.
Approval of #Aducanumab for #Alzheimer Disease—the @US_FDA's Perspective
…When the patch is worse than the hole…
Regulatory agencies cannot prioritize subjective feelings over data. That's it…@JAMAInternalMed https://t.co/tUAfq5xvNl
— Matteo Cesari (@macesari) July 14, 2021
The response from HCPs on Twitter was rather unanimous as they considered all the data and surrounding events and conversations, often calling out specific individuals for endorsing the decision. A psychiatrist in the UK, Robert Howard, wondered whether there was a parallel Twitter world with “joyful celebrations”.
I’m just wondering if there’s a parallel Twitter world in which people are tweeting their appreciation of the FDA’s excellent aducanumab approval and joyfully celebrating that their patients are already benefiting from access to aduhelm?
— Robert Howard (@ProfRobHoward) July 17, 2021
January 11th: Medicare access restriction
After months of unfolding controversy, Medicare, the US federal health coverage program, announced they would be restricting access to Aduhelm for only patients in clinical trials (and only those randomised controlled trials approved by the agency).
Medicare concluded that Aduhelm needs to clearly demonstrate cognitive or functional benefits to be paid for outside of randomized trials. FDA's accelerated approval program is intended to enable patient access without such evidence. This disconnect needs more exploration. https://t.co/if4jLRUrGq
— Sean Tunis (@SeanTunis) January 12, 2022
Before this announcement, many hospital systems (not to mention individual HCPs) made clear they would not be prescribing nor administering the drug. US Cardiologist C. Michael Gibson shared this rejection by Cleveland Clinic and Mount Sinai hailing it “one of the starkest signs of concern” over the approval. He used a Twitter poll to gauge reactions; the majority of the 141 respondents agreed with the decision due to lack of efficacy:
Do you agree with Cleveland Clinic’s and Mt. Sinai’s decision to not administer the drug #Aduhelm to Alzheimer’s patients? Why or why not?
— C. Michael Gibson MD (@CMichaelGibson) July 15, 2021
By definition, Medicare only covers products that are “reasonable and necessary”, differently to the requirements of an FDA approval when a product must be “safe and effective”. Safety concerns spiked when an AD patient died from a side effect that could be linked to Aduhelm called “amyloid-related imaging abnormalities” (ARIA).
1/n – Report of patient death, perhaps due to ARIA associated with #aducanumab (Aduhelm), highlights safety concerns.@US_FDA should require a REMS that includes regular MRIs and monitoring for ARIA symptoms.#GeriTwitterhttps://t.co/zm0cOEzJDe
— Art Walaszek (@artwalaszek) November 22, 2021
The decision for Medicare to restrict access came after a myriad of conversations about the price tag attached to Aduhelm. Biogen had initially priced the drug at $56,000 for an average dose, but later slashed the price in half to around $28,000. Dr Henry Rosenberg used this price cut to explore the wider issue of drug pricing, calling for a bottom-up approach.
A new Alzheimer's drug that may work, when released cost $56,000 a year. To show the arbitrary nature of drug pricing, the company lowered the price to $28,000 b/c people were not buying. What we need is bottom up drug pricing not let's see what we can get away with pricing.
— Henry M. Rosenberg (@DoctorHenryCT) January 11, 2022
A physician in Toronto, David Juurlink, brought the scenario to real life when he postulated that he wouldn’t prescribe such an expensive, side effect prone intravenous drug to an AD patient with mild symptoms.
Trying to envision a scenario in which I encourage a patient with mild Alzheimer's to take a drug that's given intravenously every 4 weeks, costs $56,000+ per year, sometimes causes cerebral edema and microbleeds, and we have no idea if it works.
— David Juurlink (@DavidJuurlink) June 8, 2021
A holistic perspective
Zooming out on the conversation, the pricing issue extended to expose areas of need that could be improved with additional financial investment. Geriatrician Aroonsiri Sangarlangkarn stipulated that a better way to spend $56,000 for an AD patient would be to pay a more adequate wage to caregivers.
What would you do for patients with dementia if you have $56,000/year, not including costs of PET scans or surveillance MRI?
I'll go first
Pay caregiver a wage they deserve so that every patient has adequate coverage with minimal turnover#Aducanumab
— @[email protected] (@AroonsiriMD) June 9, 2021
World Alzheimer’s Day saw over 1,000 HCP posts on September 21st with an increased dialogue about recognising AD symptoms and ramping up support for those affected by the disease. Many HCPs used the day to break down the different facets of AD from diagnosis to treatment.
Ruminda H. Gunaratne wrote a tweetorial in which he answered the question of how to treat AD, making it clear there is no cure, but cholinesterase inhibitors such as donepezil may have some benefit.
6. How to treat dementia ?
This depends on the cause of dementia. But currently in #Alzheimers disease there is no cure.
Some may benefit from drugs such as cholinesterase inhibitors (#Donepezil). But its more about the non drug management including caregiver care.
— Ruminda H. Gunaratne (@Rumindahg) September 21, 2021
Two other drugs that are in development for AD are donanemab and gantenerumab from Lilly and Roche respectively. Donanemab has earned positive phase II results while gantenerumab has received FDA breakthrough status despite missing its primary endpoint in phase II/III trial. HCPs are wary and keeping a close eye on data as these two new drugs are “amyloid clearing” just as Aduhelm.
It’s like deja vu all over again. FDA gives breakthrough status to gantenerumab, another AD drug abandoned because of proven futility. AND it’s even harder to say and spell than aducanumab. We have completely lost the plot in AD therapeutics development. https://t.co/FFpNbXPeVP
— Robert Howard (@ProfRobHoward) October 9, 2021
What HCPs Think
Aduhelm’s approval has shown how impactful HCP opinions are and a successful approval does not equate to a successful launch. Despite a wish for an AD cure, HCPs will not sacrifice their obligation for sound safety and efficacy data before approving a treatment for their own prescribing. The unravelling of events was able to be tracked in HCP online conversation, revealing where there were needs and opportunities to support patients in a tangible and effective way. CREATION.co provides HCP insights and tracking services that can keep a finger on the pulse of drug approvals and launches, in order to understand what HCPs think.
This article was first published on Pharmaceutical Market Europe (PME).
 By Mary Kangley
By Mary Kangley