13.02.2024 | Insight
What eHCPs think about 2024 clinical trials for pharmaceuticals and digital therapeutic tools
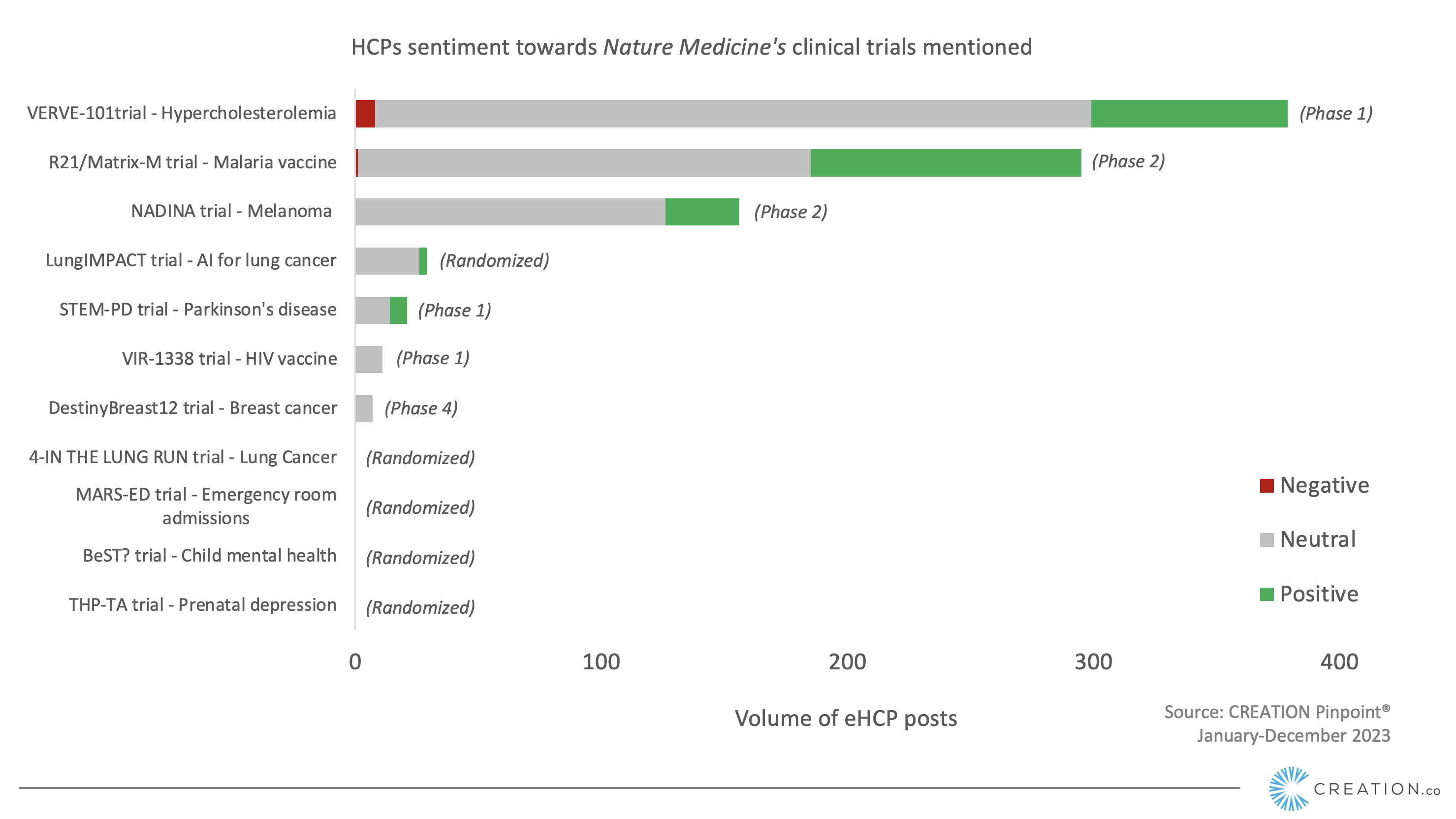
On 7 December 2023, Nature Medicine published a yearly review entitled “11 clinical trials that will shape medicine in 2024”. Among the 11 clinical trials discussed, three are in Phase 2 or higher and have already published results, with the rest are still in early stages. Most of the clinical trials were focused on pharmaceuticals, however, a few were assessing digital tools for medicinal purposes, including artificial intelligence. CREATION.co analysed how online healthcare professionals (eHCPs) responded to these trials last year.
eHCPs are eagerly anticipating the new malaria vaccine
Clinicians in the University of Oxford and Serum Institute of India are midway through a phase III trial of the R21/Matrix-M vaccine against clinical malaria in African children. This trial is coming to an end, and vaccines are likely to be rolled out worldwide soon, eHCPs have already expressed their enthusiasm about the vaccine being approved in Ghana and emphasised the importance of the vaccine, mentioning its efficacy and cost-effectiveness. eHCPs brought their personal stories around the need for the vaccine.
The new cost-effective R21/Matrix-M malaria vaccine is recommended by the @WHO and is effective in 70-80% of cases. It could change the course of malaria-driven deaths, saving 450,000 lives per year, and must receive swift prequalifications. https://t.co/T9ZEnISiWL
— Dr. Kelly Henning (@drkellyhenning) December 19, 2023
According to eHCPs, the NADINA trial is shaping the melanoma space
The NADINA trial, taking place in the The Netherlands Cancer Institute, is an international phase III trial that aims to compare the efficacy of neoadjuvant ipilimumab plus nivolumab with that of standard adjuvant nivolumab in macroscopic stage III melanoma. 44 eHCPs shared the initial results of the trial with their peers which were presented at AACR 2023. eHCPs congratulated the different institutes that collaborated on this study and the research teams. Professor Georgina Long, who was part of the research team, commented on how proud they are to be part of this study and mentioned there was more work to do. Dr Jason Luke described the results as field-changing and felt that NADINA is a “hugely important study”, in a post shared 20 times by his peers.
Field changing @SWOG 1801 #melanoma @NEJM @DrSapnaPatel showing neoadj > adj PD1. Amazingly, as many Q's as answers. Certainly IIIC/D/IV NED get neoadj but more than 1 dose? Should we give combo's #NADINA? Do we need any Rx post-surg? Hugely import study!https://t.co/FjtQhOnfgv pic.twitter.com/9zXADPK0tl
— Jason Luke, MD, FACP (@jasonlukemd) March 2, 2023
The VERVE-101 trial uses leading-edge technology for hypercholesterolemia
During the American Heart Association Conference various cardiovascular trials were presented, giving the opportunity to eHCPs to share their thoughts. One of those trials was VERVE-101, by Verve Therapeutics, a novel CRISPR base editor tool designed to alter the genetic code of patients with familial hypercholesterolemia and cardiovascular disease.
Dr Ritu Thamman described the trial outcomes as a scientific milestone and congratulated Verve Therapeutics for their achievement. Some eHCPs expressed an interest in hearing more about the trial and most were positive about the results of VERVE-101. In addition, 56 eHCPs shared Sek Kathiresan’s post, CEO of Verve, who applauded the trial as a scientific innovation. However, seven eHCPs shared Venk Murthy’s post expressing concerns about certain cardiac complications that occurred during the study and suggested that the study should terminate earlier than planned.
Results of the heart-1 study of VERVE-101 by $VERV had serious concerning cardiac complications.
May be time to stop trials on this agent for now. Here is my thinking:https://t.co/HL8pj7N9Vq
— Venk Murthy MD PhD (@venkmurthy) November 13, 2023
The STEM-PD trial receives support from eHCPs
A leading Parkinson’s therapy involves transplanting dopamine-producing cells into the brain, which could offer a breakthrough in repairing the damage. The STEM-PD trial, by the Skane University Hospital, studies the effect of implanting dopaminergic neurons from human embryonic stem cells in patients with moderate Parkinson’s disease. eHCPs were filled with hope by the potential of this trial as they shared updates online. They described this trial as a “milestone trial” after the first patient successfully received the transplant. eHCPs discussed the treatment as a breakthrough in Parkinson’s space and congratulated the medical team for their landmark contribution.
Milestone in #Parkinsons reached. First #stemcell-based #transplant with STEM-PD carried out in patient in Sweden. Agnete Kirkeby from reNEW led pre-clinical development of cell product https://t.co/UdRwIqBhAg
#stemcellresearch #brain @Kirkeby_Lab @Lund_Stem pic.twitter.com/KbEOMGCHRZ— reNEW (@reNEW_Global) February 28, 2023
Although in the early stages, the VIR-1338 HIV vaccine trial is receiving positive reactions from eHCPs
A preventative HIV vaccine is an ongoing pursuit in the scientific community to protect individuals and communities from the virus. The VIR-1338 trial, by Vir Biotechnology Inc., is testing a novel vaccine that induces an HIV immune response in people. eHCPs were delighted to receive the news that the trial has begun enrollment in the US and South Africa. Of the 110 eHCPs who shared the Nature article, 30 chose to highlight the HIV vaccine trial to comment on.
Leading researchers named their top clinical trial for 2024.
These include base editing, a vaccine against HIV, artificial intelligence tools for lung cancer, and patient triage.
https://t.co/OZlgwhYUKQ— Raja Adnan Ahmed (@drraja_) December 30, 2023
Related content
https://creation.co/knowledge/how-ehcps-responded-to-the-hiv-landscape-in-2023/
https://creation.co/knowledge/how-ehcps-can-influence-the-hiv-epidemic-in-south-america/
—————————————
Digital therapeutic and AI tools emerge
The ascent of digital therapeutics marks a transformative shift in healthcare, employing innovative technologies for evidence-based interventions. From mental health apps to wearable devices, these innovations empower individuals, offering personalised solutions. As regulatory support grows, the integration of digital therapeutics promises a more accessible and patient-centric era in healthcare.
Among a variety of digital tools currently being developed, Nature’s list included a few, including a particular AI tool for lung cancer detection.
Early detection of lung cancer is crucial for saving lives, yet it is often diagnosed at advanced stages. Aiming to expedite diagnosis and improve outcomes, an ongoing trial in six UK hospitals involves applying artificial intelligence to chest X-rays immediately upon capture. The study examines whether this AI intervention shortens the time of a patient receiving a CT scan. A few eHCPs shared this clinical trial with their peers and showcased curiosity by sharing Qure.AI’s posts, the company that manufactures the digital tool, eHCPs discussed the trial by sharing a post mentioning that it could save thousands of lives every year. eHCPs also shared posts from an NHS social media account, discussing the AI technology and the potential it has for reducing treatment delays.
Nature Medicine gives shout out to Prof @DRBLUNGS and the LungIMPACT trial as one of the top global clinical trials shaping medicine into 2024!
Utilising @qure_ai’s qXR AI for Chest X-rays, it will gather real-world evidence to test the value of AI in shortening the time to a CT…
— Qure.ai (@qure_ai) December 12, 2023
Stay up to date with HCPs’ views
As research keeps progressing, clinical trials will populate and forever alter every aspect of medicine and human health for the better. Moving forward into the digital era, more and more clinical trials may access treatments based on technology and artificial intelligence.
CREATION.co publishes monthly updates on pharmaceutical approvals and is bringing insights on what eHCPs across the globe think around the latest product launches.
If you would like to find out more about this research or connect with your healthcare professional stakeholders in a meaningful way then do not hesitate to get in touch as we’d love to hear from you and share more examples with you.
 By Alexandra Maria Chatziioannidou
By Alexandra Maria Chatziioannidou 

