CREATION Pinpoint’s Drug Launch Tracker shows how healthcare professionals (HCPs) respond to drug launch news, as it happens. The FDA approved over 940 submissions between November 1 – December 10, which included medicines for sickle cell disease, non-small cell lung cancer (NSCLC) and partial-onset seizures.
The most mentioned FDA approvals by HCPs online
| Brand | Compound(s) | Manufacturer | Brand mentions | Compound mentions | Total mentions |
|---|---|---|---|---|---|
| Oxbryt | voxelotor | Global Blood Therapeutics Inc | 16 | 32 | 48 |
| Tecentriq | atezolizumab | Genentech | 2 | 36 | 38 |
| Givlaari | givosiran sodium | Alnylam Pharms | 32 | 0 | 32 |
| Xcopri | cenobamate | Sk Life Science | 7 | 23 | 30 |
| Fetroj | cefiderocol sulfate tosylate | Shionogi | 28 | 0 | 28 |
| Abrilada | adalimumab-afzb | Pfizer | 14 | 14 | 28 |
| Brukinsa | zanubrutinib | Beigene | 4 | 18 | 22 |
| Calquence | acalabrutinib | AstraZeneca | 3 | 17 | 20 |
| Adakveo | crizanlizumab-tmca | Novartis | 15 | 2 | 17 |
| Reblozyl | luspatercept-aamt | Celgene | 13 | 3 | 16 |
| Amiodarone | amiodarone hydrochloride | Sandoz | 12 | 0 | 12 |
| Ziextenzo | pegfilgrastim-bmez | Sandoz | 5 | 5 | 10 |
| Apremilast | apremilast | Dr Reddys Labs | 6 | 0 | 6 |
| Allopurinol | allopurinol | Vintage Pharms | 4 | 0 | 4 |
| Toujeo | insulin glargine recombinant | Sanofi Us Services | 3 | 0 | 3 |
| Exservan | riluzole | Aquestive Therap | 2 | 0 | 2 |
| Reditrex | methotrexate | Cumberland Pharms | 1 | 0 | 2 |
| Stelara | ustekinumab | Centocor Ortho Biotech | 1 | 1 | 2 |
| Fulvestrant | fulvestrant | HBT Labs | 1 | 0 | 1 |
| Gabapentin | gabapentin | Vistapharm | 1 | 0 | 1 |
| Memantine | memantine hydrochloride | Sun Pharma | 1 | 0 | 1 |
| Exem | air polymer-type a | Giskit B.V. | 1 | 0 | 1 |
| Oxycontin | oxycodone hydrochloride | Purdue Pharma | 1 | 0 | 1 |
| Depakote | divalproex sodium | AbbVie | 1 | 0 | 1 |
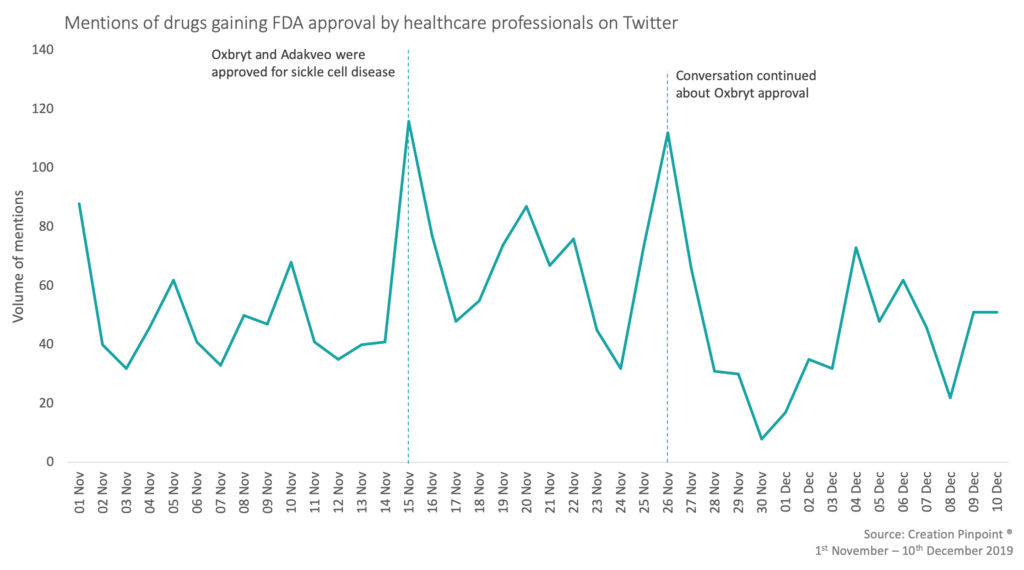
The most mentioned approval this month, with 48 mentions, was Global Blood Therapeutic’s Oxbryt (voxeltor). This was approved for the treatment of sickle cell disease (SCD) in adults and children 12 years old and older. A Canadian physician called the approval of Oxbryt, along with crizanlizumab, a ‘New era of hope’ for people living with SCD which was reposted by five other HCPs with others also being positive saying that it is ‘Good news’ and ‘breakthrough therapy’.
Within 2 weeks, the FDA has approved 2 new treatments for SCD- crizanlizumab (as monthly infusions) and voxelotor (as daily oral tablets). A new era of hope has dawned for people living with SCD.
— Isaac Odame (@DrOdame) November 25, 2019
But among the positivity there were five HCPs that expressed that they couldn’t believe it had been approved with ‘Unvalidated surrogate endpoint’.
The second most mentioned product was Genentech’s Tecentriq (atezolizumab) for the treatment of NSCLC. HCPs shared news referencing the IMpower130 clinical trial and the data that led to the approval. Stephen Liu’s post was retweeted six times by his peers, while the product was mentioned 38 times in total by HCPs over the period.
#OncoAlert FDA approves combination of carboplatin, nab-paclitaxel, and atezolizumab for advanced, non-squamous #NSCLC. Approval based on IMpower 130 which showed an improvement in both PFS (HR 0.64) and OS (HR 0.79) compared to carbo/nab-pac alone. #LCSMhttps://t.co/Z95qpIXXyO
— Stephen V Liu, MD (@StephenVLiu) December 4, 2019
HCPs were also positive towards the approval of Givlaari (givosiran sodium) by Alnylam saying that this is a landmark event for Alnylam and that this is a ‘big day’. Five HCPs shared the Alnylam post celebrating the news of the new approval.
We are thrilled to share the news that we have received @US_FDA approval for GIVLAARI (givosiran), the second #RNAi therapeutic from Alnylam approved by the FDA in less than 2 years. #raredisease #biotech
— Alnylam Pharmaceuticals (@Alnylam) November 20, 2019
We are tracking the HCP reaction to FDA approvals and related topics each month. You can keep up to date with this and other pharmaceutical tracking updates within the Tracking section of CREATION Knowledge, or by signing up to our monthly eJournal.
READ LAST MONTH’S FDA APPROVAL TRACKER:
‘A new hope’ found in the FDA approvals of Entresto and Farxiga
Methodology notes:
- Data for this research was analysed from the online Twitter conversations of HCPs talking about FDA approvals in English language (all other languages are available), between November 1 – December 10 2019.
- Between November 1 – December 10 2019, 1,519 HCPs shared posted about FDA approvals 2,099 times all over the world.
- The table refers only to approvals that occurred between November 1 – December 10 2019. Mentions of approvals before November 1 were not included.
- HCPs mention brand and compound names when discussing FDA approvals. Approvals may include multiple compounds.
