11.04.2024 | Tracker
Product Launch Tracker: eHCPs celebrated the first approved treatment for patients with NASH
Every month, CREATION.co’s tracking updates bring you the latest insights from the online conversation of healthcare professionals (eHCPs) across the globe discussing product launches. Discover which new drug approvals eHCPs are talking about, what they think about them, and which online sources they are using to inform their opinions and conversations in CREATION.co’s latest tracking update.
———
Throughout March 2024 CREATION.co tracked the global conversations of 1688 HCPs who posted 2577 tweets about the launches and approvals of new products.
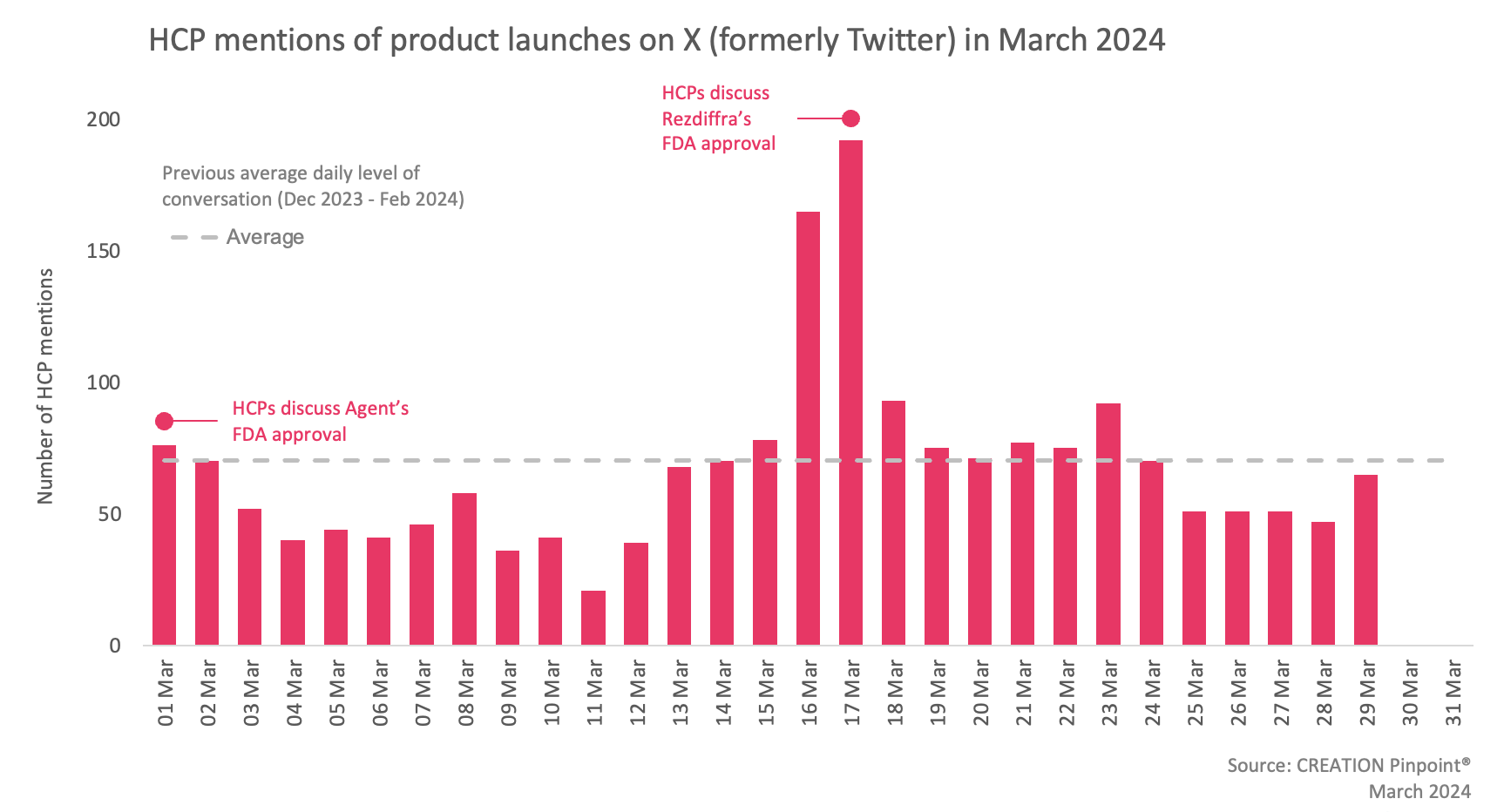
In March, 277 more eHCPs discussed treatment approvals than in February, publishing 23% more posts during that time.
On 14 March, the FDA approved Madrigal Pharmacautical’s Rezdiffra (resmetirom) for the treatment of adults with noncirrhotic non-alcoholic steatohepatitis (NASH) with moderate to advanced liver scarring. Online HCPs were thrilled about the approval as it is the first treatment approved for NASH. It represents a breakthrough for patients and offers them hope, said Dr Jan Kral, a gastroenterologist. Hepatologist Dr Naim Alkhouri characterised the day of the approval as a ‘historic day for the NASH space’. Many eHCPs celebrated the news calling it a ‘hurrah moment’ for patients and bringing the promise of much progress to come.
Firsts are worth celebrating. In this case, the cause for celebration is especially great. Late this week, @US_FDA approved #resmetirom to be the first ever treatment for #MASH with #fibrosis.
This brings the promise of much progress to come.https://t.co/r5lqX44K1x— Ted Kyle (@ConscienHealth) March 16, 2024
NASH field celebrates ‘hurrah moment’ with a first FDA drug approval for the liver disease! Rezdiffra is approved by US FDA https://t.co/7ssepEy13q
— Dr RH Jani – ॥ गुरु ॐ॥ (@rhjani) March 19, 2024
On 8 March, the FDA approved a new indication for Novo Nordisk’s Wegovy (semaglutide) injection to reduce the risk of heart attack and stroke in adults with cardiovascular disease and obesity or overweight. Most eHCPs were pleased with the new indication approval, calling it “a big win”. Other eHCPs such as Dr Matthew Bowdish posted about the positive outcomes of the SELECT trial. A few eHCPs also commented the new indication will improve and expand insurance coverage for weight loss treatments.
BIG NEWS:
Novo Nordisk's #Wegovy wins FDA approval for cutting heart disease risks in overweight patients in move that could expand insurance coverage https://t.co/fY2BRRnO1i
— C. Michael Gibson MD (@CMichaelGibson) March 8, 2024
A big win for Wegovy: Weight loss drug semaglutide first in history to gain FDA approval for reducing heart risks https://t.co/EotSLHdUMl
— David E. Albert, M.D (@DrDave01) March 16, 2024
On 1 March, the FDA approved Boston Scientific’s Agent paclitaxel-coated balloon, the first such balloon to be approved for the treatment of coronary in-stent restenosis. A few eHCPs who shared the news expressed their enthusiasm for the new treatment. They also highlighted the benefits of the new treatment compared to conventional angioplasty and discussed the positive outcomes of the trial with their peers.
In the first US trial evaluating a drug-coated balloon for coronary in-stent restenosis, we found that the Agent paclitaxel balloon reduced target lesion failure by > 40% compared with conventional angioplasty.
Now FDA approved.
Published in JAMA now:https://t.co/DyAsIl3TQk
— Robert W. Yeh MD (@rwyeh) March 10, 2024
🔥So excited to hear that FDA just approved Drug Coated Balloons for coronary use (ISR) in the United States (finally !!) 👏#CardioTwitter #Cardiology #ACCFIT #CardioEd @US_FDA @BCMHeart @Pooh_Velagapudi @adityadoc1 @JingLiu_MD @AkuCardiology @minhaskh https://t.co/Xz9gKr9xP4
— Umair Khalid (@Umair2017) March 1, 2024
The three most shared stories from eHCPs discussing product launches in March were:
- A JAMA Network’s article on the approval of Agent, a paclitaxel-coated balloon for the treatment of coronary in-stent restenosis.
- An FDA press release on the approval of Wegovy, for reducing the risk of cardiovascular disease for adults with obesity.
- An FDA press release on the approval of Rezdiffra for the treatment of adults with NASH.
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area-specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- CREATION Pinpoint® listened to the discussions amongst online HCPs of pharmaceutical product launches and drug approvals between 1 March and 31 March 2024.
- The data included mentions of drug approvals by the FDA, EMA, NICE, and CHMP, as well as HCPs’ use of the phrase ‘drug approval’ in their tweets.
- Between 1 and 31 March 2024, 2517 online HCPs worldwide made 1688 references to new pharmaceutical product launches and drug approvals.
 By Alexandra Maria Chatziioannidou
By Alexandra Maria Chatziioannidou 

