Throughout March 2023 we tracked the global conversations of 2,254 eHCPs who posted 3,228 English-language Twitter posts about the launches and approvals of new products.
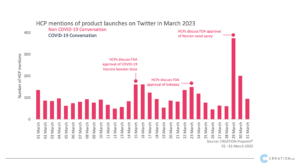
In March, several product approvals were discussed online by HCPs. They offered their thoughts on a novel treatment for Friedreich’s ataxia, approved migraine products, as well as opinions on Pfizer’s fourth doze booster COVID-19 vaccine.
One of the first approvals which generated significant conversation among HCPs was the FDA approval of Skylarys (omaveloxolone), as the first treatment for Friedreich’s ataxia. It is a rare genetic disease that damages the nervous system of the individual and impairs their muscle movement and speech. HCPs shared the approval news, with some seeing the approval as an important step forwards as the treatment is the only one to ever be approved for that specific disease. According to HCPs with this approval the FDA widens the path for rare disease treatments’ new approvals and quoted “we’ll be see many more therapies for neurological diseases in the near future”.
FDA approval for "Skyclarys, First Ever Treatment for Friedreich’s Ataxia". We'll be see many more therapies for neurological diseases in the near future. Exciting time. https://t.co/ixcqZB4rZ2
— Sunil Thummala MD (@TX_neurologist) March 1, 2023
In an important step forward for the Friedreich’s ataxia community, the #FDA has approved the first therapy to treat #Friedreichs #ataxia. Reata Pharmaceuticals’ SKYCLARYS was approved on #RareDiseaseDay2023. Read our news story to learn more https://t.co/msuX89bEpX pic.twitter.com/J6o7qKuJVL
— Oxford-Harrington Rare Disease Centre (@OHRareDisease) March 8, 2023
On the 14th of March an important COVID-19 update triggered the HCP online conversation. FDA approved Bivalent Pfizer-BioNTech COVID-19 vaccine as booster dose for certain children 6 months through 4 years old. This approval drew criticism from HCPs, as they mentioned that it is unnecessary for infants and “simply obscene”. Generally this FDA approval generated negative comments from HCPs and was compared with regulations from other countries.
Towards the end of the month, EMA recommends approval of Bimervax as a COVID-19 booster vaccine for people aged 16 years and above who have previously been vaccinated with mRNA COVID-19 vaccine. This approval was more neutrality discussed, as HCPs mainly shared the authorisation news.
‘Simply Obscene’: FDA Approves Fourth COVID Shot for Infants and Kids Under 5 • @ChildrensHD
This latest FDA move, draws criticism from Physicians that it’s not needed, lack of #InformedConsent, and widespread #SideEffects.#CourageousDiscourse
https://t.co/0hbRHwUCM7— Craig M. Wax D.O. (@drcraigwax) March 16, 2023
EMA recommends authorising HIPRA's #COVID19vaccine: Bimervax 💉, as a booster in people aged 16 and above.
🔗 For more info follow this link: https://t.co/vpXoArENGd#COVID19 #Vaccination #UnitedInProtection pic.twitter.com/o5GIfiWZcG
— EU Medicines Agency (@EMA_News) March 30, 2023
HCPs also shared their thoughts on FDA’s approval of fast-acting nasal spray spray to treat migraine under the name Zavzpret (zavegeoant) from Pfizer. It is the first nasal spray of CGRP inhibitors to be approved by the FDA for migraine treatment. HCPs were quick to share the approval news, where they posted optimistic reactions around it. HCPs congratulated the manufacturer and shared the trial results which were positive.
Excited for this new nasal spray #gepant abortive! 👃 #Zavegepant #Zavzpret #migraine #headache #chronicmigraine #NeuroTwitter #Neurology #Neuro #MedTwitterhttps://t.co/BulzZQUs9V
— Dr. Eric P. Baron (@Neuralgroover) March 10, 2023
Congrats to @pfizer for the @ApprovalFda of #Zavagepant #Zavzpret a new #acute #treatment 4 #migraine! Studies demonstrated fast-acting w some results in ~15mins. Another anti-CGRP novelly delivered (nasally) #CGRP I hope it is made accessible for all patients. #fda #healthequity
— L. Charleston IV, MD, MSc, FAHS (@LCharlestonIVMD) March 11, 2023
On March 22nd, the FDA approved a antifungal used for the treatment of Candidemia and invasive candidiasis called Rezzayo (rezafungin) by Cidara Therapeutics. Invasive candidiasis is a serious infection caused by a yeast (a type of fungus) called Candida that can affect the blood, heart, brain, eyes, bones, or other parts of the body. HCPs were enthusiastic about the approval as candidemia and invasive candidiasis had very limited therapy options, with the last medication having been approved more than a decade ago.
🔥 BREAKING 🔥
U.S. FDA approved REZZAYO™ (rezafungin inj) novel once-weekly echinocandin for the treatment of candidemia & invasive candidiasis in adults with limited or no alternative treatment options #IDTwitter @FungalDoc@IDstewardship @InfectiousDzhttps://t.co/u3tuTPqIQq— Antibiotic Steward Bassam Ghanem 🅱️C🆔🅿️🌟 (@ABsteward) March 22, 2023
🎉New FDA Medicine Approval
⚕️Medicine: Rezzayo
💊Active ingredient: rezafungin
✔️Indication: to treat candidemia and invasive candidiasis
⚙️Mechanism: an echinocandin that works by inhibiting the enzyme, 1,3-β glucan synthase
💉Once-a-week injection
— PharmaFactz (@PharmaFactz) March 26, 2023
Emergent BioSolutions recently announced that the FDA has approved Narcan (naloxone) nasal spray for over the counter emergency treatment of opioid overdose. This medication has been characterised as life-saving and will be widely available without prescription. HCPs were enthusiastic about this approval as drug overdose persists as a major public health issue in the US. The nasal spray’s approval was described as “a step in the right direction” and a “historic milestone”.
Today, U.S. FDA approved Narcan, 4 milligram (mg) naloxone hydrochloride nasal spray for over-the-counter (OTC), nonprescription, use! ❤️ https://t.co/A7vEYD44oz ❤️ Paves the way for this life-saving medication to reverse an #opioid #overdose to be sold directly to consumers in… https://t.co/BM79ir0B8z
— Jim Dwyer MD (@JimDwyerMD) March 30, 2023
Today, the US FDA approved Narcan (naloxone) nasal spray for over-the-counter, nonprescription, use — the first naloxone product approved for use without prescription.
This is huge. Naloxone saves lives.
I hope this momentum continues to other policies! https://t.co/q88ez70mxe
— Ryan Marino, MD (@RyanMarino) March 29, 2023
The three most shared links from HCPs discussing product launches in March were:
- A news article from Globe Newswire on the FDA approval of Rezzayo for the Treatment of Candidemia and Invasive Candidiasis.
- A New York Times press release on the FDA approval of Nacran, a nasal spray which reverses opioid overdoses.
- An FDA press release on the approval of first over the counter nasal spray which reverses opioid overdose.
Each month, CREATION.co tracks the HCP conversation relating to new product launches.
You can keep up to date with this and a variety of other topics including virtual congress, healthcare changes since the pandemic, product development and therapy area specific insights within the Tracking section of CREATION Knowledge, or sign up to receive our monthly eJournal with all of our latest HCP insights.
To stay up to date, you can sign up to CREATION.co’s monthly eJournal.
Methodology
- Using CREATION Pinpoint® the English-language Twitter conversations of HCPs globally discussing new pharmaceutical product launches and drug approvals between 1 March and 31 March 2023 were analysed in order to discover which new product launches HCPs are discussing as well as #WhatHCPsThink.
- Mentions of drug approvals by the FDA, EMA, NICE, and CHMP were included in the data, as well as mentions of ‘drug approval’ by HCPs in their Twitter conversations.
- Between 1 March and 31 March 2023, there were 3,228 HCP mentions of new pharmaceutical product launches and drug approvals from 2,254 unique HCP authors from around the world.
Click here to read the latest Product Launch Tracker
 By Alexandra Maria Chatziioannidou
By Alexandra Maria Chatziioannidou 

